Sat. 30.05 - decided to 'feed' some less to start seeing a difference in behaviour
Sun. 31.05 - because I don't want to much moisture to build up (might lead to bacterial mold) and to keep caring well for the Physarum & first get a better understanding of it, I gave each dish a full spoon of oats today.
Sun. 31.05
Starting to notice this white-ish slime. First I thought it was mold but now I think it is the trace that Physarum leaves behind itself - which if it has enough space hinders it from going to this spot again. Might be having a strange reaction with the Agar?
Sun. 31.05
Seeing an answer to the question: does physarum grow on surfaces that are not Agar: yes!
Does it maybe draw the moisture from the surface of agar so that it doesn't need it in other/all areas?
Sun. 31.05
Also you can start to see the digested oats that are no longer yellow so the blob moved off of them because they offer no more for it.
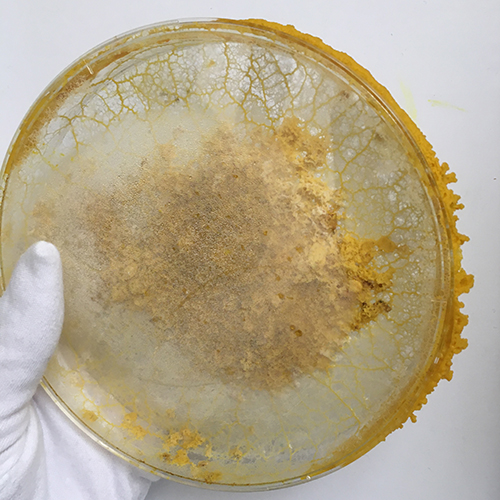
Mon. 01.06 - The first petri-dish, probably older than a week now changed colors, it doesn't look like mold but it's clearly much much darker then if was yesterday.
Also doesn't look like the other phases of the life cycle?
Mon. 01.06 - Here you can see Physarum build a squiggly mass on top of the oats, it's more like a pile of yellow little blobs than the vein like structure.
Mon. 01.06 - Also there is a lot more moisture in the dishes again - maybe today is finally a day to inoculate?
What isen't really clear to me is what to do with the old dishes from which I inoculate new ones? Do I let them go their course? Do I try to transfer as much of the blob and then let the leftovers dry?
Answer - Mon. 08.06: I wait until the dish is fully dried out our possibly contaminated to throw away whole dish in worst case or clean out the already dried Physarum. I now have pre-made petri-dishes for when a lot tries to escape. If only a little tries to come out I put it back in the same dish.
Observational notes
1 large petri dish the agar never settled even though it is the same mix as the others- possible because I moved it too soon disrupting the cooling process?
Also I need to make sure I have a bit more Agar next time as the solution is very wet in general.
The petri-dishes with the discarded oats and Physarum clearly show that Physarum senses no more food and is trying quickly to escape to survive somewhere else.
Also the medium older dishes show me that Physarum has a sense for the environment and tells me very clearly it wants a new home! Even with enough food it wants to move on somewhere with better conditions - within one night it's climbed half way out the lid.
The others are quite happy where they are though.
The small dishes - after I removed the excess /old food seem 'happy' - they've reconnected and are staying inside the dish, also no colour change but the structure looks like it might be going towards spores.
I'm getting a sense for how Physarum perceives it's environment slowly.






The Physarum's structure is obviously much better visible when NOT ON OATS - so what are aesthetic and sustainable ways to replace the oats or make the food source disappear?
A new inoculation follows!
A comparison between two dishes to show the change in colour of the eldest to how it 'should' usually look.
Mon. 01.06 - Interesting to see the structural difference between the digested oats and the fresh ones quickly being covered by Physarum.
Mon. 01.06 - Also other dishes are starting to form black dots which I can see more clearly from the bottom - which show bacterial/Yeast contamination and indicate that I should change the dish
Mon. 01.06 - Clearly the Physarum wants out, but isen't even healthy enough to make it.
Day 1 - freshly transplanted
I removed the oats with Physarum from the old dishes and preserved it.
Day 2 - still strong but also fast moving out of the dish.
clear indicator that the leftovers can't just be thrown away as the organism still lives on even when perceived as 'discarded' by me. Even spreading faster than on the new Agar dishes.
Day 3 - The structure already looks much weaker and the colour is more faint
Day 1 - First movements in the big dishes
All small dishes are starting to look 'healthier' again - strong structure and colouring
Also, the longer it has been in one petri-dish, the darker the color gets - maybe because it is stronger and denser? Hence also the squiggly structure that builds up? Or maybe because it continues to digest the oats?
Here, in both dishes, we can see the dish looks quite dry - in the one on the left the Physarum is crawling out because the environment is not good enough anymore.
In comparison the new dishes have a very moist agar (too moist) and are spreading out across the space.
Personal note: I like the structure when it isen't on Agar visually better ..
Also the structure of it faded and dried out is an interesting contrast.
Of all the large dishes the physarum in this one climbed out of the dish so quickly - even before exploring it's designated area and connected with the physarum from the other dish - almost like it sensed it's other half was in trouble and reached out to create a bridge.
I'm interested in this idea of distance - of sensing eachother when apart and coming back together - how far is too far? Could there be a correlation between physarum hat has recently been divided that finds back together more quickly than those that have been apart for longer?
A type of body memory?
Thurs. 04.06. - the oldest medium dishes are starting to dry up - clearly the agar is no longer viable and one tried to escape while the other is more feeble.
To my great surprise the smallest - oldest physarum are doing the best - best colour and structure
Generally I'm noticing if I give more oats I have less problem because they take up the moisture and make the dish less prone to produce things I don't want.
I'm still at a loss for what to do with dishes that I've inoculated from - do I let them dry - do I keep giving them oats until they try to escape and then dry up - if it's dried in the dish do I have to throw the whole thing away?
What about the traces that show up when dealing with the dishes - scraping the escaping physarum?
Can we talk about creating a special medium?
I had these liquidy membrane looking blob parts - what are they? A weird form from the Agar which was too liquid?
Answer: When compared to the freshly inoculated dish: these are the spots of the Physarum placed - it moved from this spot quickly on to the fresh oats and these spots might have had a strange reaction with the still too fresh Agar or brought rests that where no longer good for Physarum and turn into this weird blob.
Notes from session:
Needs to be fresh and WITHOUT old foodsource
with too much oat flakes it might grow to strong and build spores
The aim: Understand the organism to then use it in artistic means.
The longer the Physarum is with me the more it changes in each dish differently and the more I feel the need to understand its lifecycle.
Spores: the sacrifice of the group to let the new generation survive
Clearly 6 days is the limit - better to change them after 5 days - like this guide also suggests: https://drive.google.com/drive/folders/1BYdpvPxnUNgdqqu1ELHTnbWlc0cQqvu6
What can I do if I see that probably bacteria is growing but not on the Physarum but in the AGAR?
Answer: Cut it out!
3/4th spiritus
1/4th water >> 70% solution
200ml - 3,4 - 4 g Agar
don't put stuff back because you don't want to contaminate your main source
Melina's way: Bring water to a boil once - add agar - then let it cool
How can something be one organism - unicellular - but have a body with different ages?
In one of the small dishes Orange slimy blotches started forming: Orange bacteria - might be a pathogen - get rid of it/leave the dish alone and just observe and don't let it contaminate by inoculating from it again.
Can I keep the excluded Physarum alive without Agar but by spraying it with water?
Answer: not really: it dries and builds molds.
Thurs. 04.06 - felt like doing open heart surgery.
Instead of letting the bacteria fully take shape which I could see through slightly milky blotches on/in the Agar (especially in places untouched by Physarum) two of which started also forming the tiniest of black dots ( which I could see will turn into bacteria 'infection' of the dish) I decided to take the scalpel and cut it out before it could fully spread and contaminate the Physarum itself.. lets see if this prevents it. But I couldn't just watch and let it happen.
Thurs. 04.06 - I've decided to not neglect the discarded Physarum on the paper towel - it has no Agar but it seems the perfect opportunity to try supplying the moisture just by spraying it once a day and giving it oats!
Fri. 05.06 - A second of the smallest dishes has turned brown - only this one has immediately started also building a white furry mold - I accidently opened it for a second and the smell from it made it clear this is not a healthy mixture of bacteria - hence I sealed it immediately with tape
The other browned one still looks the same - no mold - just drying out
Fri. 05.06 -The paper towel dishes that I decided to feed and sprits with water: of one of the the added oats are covered, the physarum has a orange, flaky consistency.
The one that originally seemed stronger has build a orange-brown-black blob that doesn't look healthy
Fri. 05.06 - Somehow I feel like Physarum is happiest in the physarum sized dishes - good thing I have more now - MADE OF GLASS will inoculate to them today
Fri. 05.06 - First day after cutting the bacteria infections out of the Agar base the dish and physarum look good! to my intense surprise - for now this intervention seems to have worked!
Fri. 05.06 - I've also decided to let the physarum crawl out of the oldest medium dishes - then dry it on the paper towels for perservation and then clean out the medium dishes for recycling/re-use
Fri. 05.06 - Also, I have the suspicion that if the dishes are touching the physarum tries to crawl out faster ..?
Fri. 05.06 - Again with 200ml water + 4g agar the solution stays liquid very long - I really think next time I need it to boil (really high) longer
Fri. 05.06 - Now looking back at my first inoculation I realise something I did not do consciously: the first time I moved the physarum with a large chunk of oats - this time I tried to take only physarum without any old oats !
Let's see how it differs because the first inoculation went very well - and also with the discarded oats I saw how strong Physarum eminated from it so maybe it is better to take with old oats - supply new and let physarum travel over and then remove the emptied old oats from the new dish ..?
Sun. 07.06 - Pro-tip: Have more dishes in the fridge than you needed so that when the physarum tries to escape the old dish you can incoluate without hassle
Sun. 07.06 - it becomes interesting on uneven structure because it layers the organic-ness
+ i find the thinn yellow even structure the most beautiful
Sun. 07.06 - I can't tell if the white slimeness comes from the agar - or slime mold agar reaction or if it is contamination of the slime mold
Sun. 07.06 - got rid of 4 dishes today - 2 of the small and 2 of the medium; the last older one with agar and the paper towel one that started building mold
They are trying to connect. It is as if the fresh one is trying to help out the old one. They are building a bridge for the organism to travel over into the new 'better' petri dish. Leaving traces of it's escape as it goes.
Loads of escaping happening from the new dishes - why is this? Does Physarum not like the new Agar?
Or is it simply stronger than it was last time?
In the older dishes, day by day it become clearer that Physarum wants out - literally. In the driest I finally decided to clean it out.
The greying blob turned into a more pronounced bacterial squish a day later
Fri 05.06 - The Day after I cut it out it looks alive and well!
Quickly it turned brown-ish as the other small dish only here clearly 'bad' bacteria and a hairy mold started forming.
I discarded this dish - along with the other dark small dish which proceeded to not change further.
Fri 05.06 - I picked the strongest dishes to inoculate from
+ IT WAS INTERESTING TO SEE THAT WHERE I SCRAPED THE PHYSARUM IN ONE DISH IT IMMEDIATELY CREATED THESE VERY LIQUID BLOBS - almost like it was bleeding
Mon 08.06 - I've gone over to only taking pictures of special traits - otherwise this is too much - documenting more than 10 dishes every day
Mon 08.06 - I find it strange that these bacterial infection dots keep showing up in the agar itself and only in some dishes ... I feel like my agar is having more bacterial infections than the organism itself
Mon 08.06 - Also I've gone over to having petri dishes ready for when physarum escpaes I'll put some back in thee same dish but I'll give some a new home immediately
Mon 08.06 - the more physarum layers over eachother the more it looks like brain or lung - liver structures
Mon 08.06 - the fight for life - even though most of the organisms body has mutated in a strange way - surpsingly a last part of it is trying to surge to life
Tues 09.06 - Definitely find it more exciting and somehow more appropriate to see Physarum on some of it's natural habitat. How are we suppose to genuinely observe this organism and understand it if we see it in conditions so far removed from nature?
Wends 10.06 - Two structural things I find intersting: the traces the Physarum leaves on the Agar
How the growing out between the cracks reminds me of the space between wood bark and tree trunk
Tues 09.06 - Clearly these dishes are turning into the brownish - green-isch colour. At this point if I didn't know about the different life cycles of Physarum I would rather think this is what happens always and not 'building spores'. Also a clear indicator that Physarum can't survive here anymore is that in comparison to the oats of the other dishes here they are still left mostly untouched.
Tues 09.06 - These are looking really unhealthy












Weeks 1 - 4
So far I have been waiting for the moment where I feel 'on top of everything', this moment eludes me still but it is time to take stock and reflect on my conduct so far.
I have been documenting in the form of photographs a lot, simply because the organism is constantly evolving so that if not captured the moment will have passed.( As initially everything was new I was taking photographs of all dishes. I have since gone over to only documenting special structures, or bacterial infections or other unusual features.) I am also writing down short notes of thoughts that struck me, my immediate observations, without analysing those thoughts further yet. My broader conceptual thoughts have been on a basic level, connecting different impressions in order to form my own perspective towards the organism.
At the same time my point of view was never entirely free as I entered this project with (1) already a interest for (especially) the dynamic, mind-of-it's-own quality of a growing organism (also relates to Fungi)- meaning a special interest in structural/visual appearance and time/movement/growth and (2) the ambition to create a 'sculptural moment' i.e. a special interest in forms for the organism to grow on.
I am starting to realise that this might result in the approach that I want to create first a setting/environment/habitat( i.e. sculpture) and then let the organism live, grow, decay on it's own accord. So, creating an interactive setting and then re-treating the human influence. This would include a process of first understanding both the organism's natural (the woods) and man-made (the petri dish) habitat to then create a hospitable setting which will allow the organism to thrive and live autonomously (as it should/could).
What is slowly becoming more and more clear is that I am interested in what I see, in the immediacy of the time I spent with the organism and the things I can observe with my own eyes. Although Physarum polycephalum is especially interesting for it's complex survival features, which have made it not only unique as a unicellular species but also one of the oldest species on earth, I don't want to develop my project based on the established scientific facts I can google about it. As I come from a background in branding were concepts emerged often on abstract conceptual implications and connections found out by reading about something, this time I want to base my work on what I, myself experience, observe, think through my interaction with the life form. (I want it to be what it is - I want to abstain from conceptualising on some invisible meta level.)
It is surprising to me that in all the research and projects I have seen related to PP no one has focused on the Biotop or habitat. Instead treating food/nutrients and hydration as isolated and therefore both scientifically concrete but organically/naturally abstract supplies given to the organism by humans, completely detached from the original, natural world. Humans who try to keep the organism in an synthetically/prosthetically prolonged state of life ' A perpetual state of life'. ( Also links to the idea of productivity - when is the organism useful to us? how is it being conformed to our systems of living? ) - by eventually retreating my human influence on my PP that has existed only in man-made petri-dishes, it would be freed from this 'perpetual state of life' - free to transform through it's natural life cycles.
It is in a way narcissistic to think about only my relationship to the organism and unobservant to not think about the organisms relationship to it's original surroundings.
The structure of the one with THE LEAST oats flakes is most beautifully visible > reaffirming the idea that for aesthetic and presentation purposes oat flakes are not the ideal food supply
Comparison: 3 newly inoculated dishes : with less food the physarum spreads faster, thinner and more 'veiny' - the colour is more faint.
Wends 10.06 - Clearly done for! I don't want to keep these anymore as they are starting to look toxic. I'm cleaning them out.
So: got rid of these 5 dishes today!
Also concluding observation: the Physarum on the paper towels with the old oats eventually starting building mold in both cases so this is neither a way of keeping alive nor of preservation for later.
Wends 10.06 - Because this Agar was looking very unhealthy I moved the entire middle part (even including a bit of it's Agar) to a new dish)
Thurs 11.06 - Miga suggested to try to recreate something I am observing. I am interested in this texture which only seems to appear sometimes - can I recreate it? figure out what stage this is or how to achieve it with certainty?
This is
an observation
a documentation
a monologe
a relationship
a research
an experiment
a controlled interaction
a perpetual synthetic state of living
a playful interaction
a caring gesture
an adoption of life
a collection of notes
a starting point for thoughts
I should observe if the dihses I take from the fridge also get these bacterial spots which I cut out ( which works really well actually- afterwards I've had no further problem)
Also still haven't figured out what the white substance is
when the slime mold has reached the stage ob being a organ turning mass that shows no structure and it is put in the same dish it might start to build these brown spots - it seems it is too old to me fully integrated back into the plasmodial organism - INDICATION OF DIFFERENT AGE IN DIFFERENT PARTS OF THE UNICELLULAR BODY - the old is rejected by the new
This is most mazing sturcture I LOVE - made from exactly these old blobs put into new dish - I should observe how this older mass reacts when put in same dish and when put in new dish
Developments:
more solid Agar is definitely better to observe the organism more isolated but I continue to wonder what it would be like to observe the organism actually free.
Also the idea of leading the organism away from an old food source in one of the bigger dishes hasen't worked. Generally I would like to see PP wander away from a spot, I've only seen it spread out from a central point in the same radius of a petri dish. But what makes PP special is it's ability to wander away from it's origin
My preferred method of inoculating:
Letting the dishes progress to the point where they dry out- often see this accompanied with a white slime and then just white colour in the dish) or change into the weird- grey-green mush (less preferable and potentially harmful I've now had a few times, and inoculate dishes from the fresh PP that climbs out. In the last sessions I've often taken blobs/lumps of just Physarum (no old oats) that was already climbing out of the dish and these have turned into strange, alien, real BLOBS ( deserving of the description of BLOB, not net like fresh PP) , some brown (even slightly colouring the Agar itself & fitting to my observations that some parts of the orang-y physarum that crawls out between the lid turns dark or even black-ish brown in spots.
From my observations I wouldn't say that PP is one big cell, as parts of the plasmodium age and are then rejected by the fresher parts of plasmodium.
One of the next inoculated dishes will get a sticker stat documents the inoculation date and I will follow the question: HOW LONG CAN YOU LIVE WITHOUT ME ?
In regards to the box I will take a picture every day at 12:00 sharp and see it's movements and I can leave the one iPhone on it to film through the glas a timelapse
von 14 petridishes haben 6 einen blob gebildet - 3 davon sind schwarz
29.06.2020 - Physarum was healthier when I gave it more oats and when I inoculated with a bit of the oats - the 'fresher' parts of it's body, not the older, outreaching arms
29.06.2020 - In the terrarium I quickly get to find that in fact : the organism travels and if given enough space moves far away and does not return to it's spot of origin. To my surprise it even splits itself and leaves parts of it's original mass behind.
01.07.2020 - insight the more 'free' agar the more bacterial problems
for now it seems that the places where physarum is it is fine - so maybe I need to make physarum spread more quickly over the agar through oats?
The more limited the space the more it just covers everything - before moving off the agar and onto the walls again
Used 1 liter of distilled water and 20g of agar agar to fill the entire glass box - a little bit less might have sufficed but the Agar spread out also clearly shows the tilt in my floor. Next time I should position it somewhere else to try to avoid this tilt and create a more even agar spread.
It is interesting to note however that it can not be clearly seen which side has less or more agar by the amount of physarum. For a day or two it way more veiny on the less agar side but generally it remains spread fairly evenly across the entire space.
Using many oats to make physarum spread across the entire glass space is great to avoid bacterial infections; generally this worked a lot better than anticipated.
I am now adding foreign objects (twigs) of different thickness and dimension to see how Physarum spreads onto and over them and if this might even effect the look of the PP structure.
*Note also: compare to non-twigs to indicate if maybe twigs still have microorganismic and bacterial 'food' on them?
Twig No1 : Spread oats around the touchpoints of PP and twigs, started in the late afternoon/evening so light was quickly very dark. By next morning: completely covered
Twig No2 : Thin twig with very thin branchings. No extra oats. Shaded daylight.
Twigs - surface: thickness
Candle - surface: material
Backhefe - food
Leaving artefact in woods for possible bacterial transfer - food
Agar cubes (as pods) - moisture
Sprayed, no Agar - moisture
Sprayed, no Agar on wax - moisture + material
Outlining Experiments
Wax and twig in glasbox
observation: since PP reached the peak of covering the entire floor/agar once it no longer needs oat flakes.
Insight: > Oatflakes are not a requirement for PP to ascend onto the wax material - I would surmise/conclude from this observation that the 'need' to find new/fresh surface/ground is bigger than the 'need' for 'food'.
I gave it fresh oat flakes on a Thursday to cover the entire floor/Agar so that no bacteria could grow there - now on a Wednesday PP is still yellow and pulsing.
Insight: fresh space is more important than fresh 'food'
> this gives me further indication that it should be possible to omit the oat flakes all together
Observation testing hypothesis:
The conditions in the box are different from the conditions in fresh air:
while PP thrives inside the box, nce the objects are taken out from the box PP vanishes/receads immediately in the room / fresh air environment.
Decision-making: It might be important that I keep control over the conditions and make the sculpture to an extend 're-creatable' by creating a closed environment?
Next step: To gain some insight/understanding into how the conditions inside and outside of the box are different from each other I could get a measuring device.
Hypothesis: Humidity - moisture in the air that rises from the agar and makes is generally damp enough so that the surface itself does not have to contain any nourishing moisture.
Observation: When touching the wax it is not damp or wet.
Comparison twig/Wood material to wax material:
test:
PP on wax outside of containment
* note also I'm not adding any extra elements like colouration and traces tested either through red beet or ph fluids 'just' sticking to the basics of plasmodial growth is difficult enough
test:
Does PP keep spreading without the connection to the Agar base on the wax?
test:
Does bacterial transfer work?